|
6
|
Atomic number (Z)
|
|
C
|
Symbol
|
|
12.011
|
Average atomic mass
|
Always look at the Legend on each periodic table to be sure of the information given in the square.
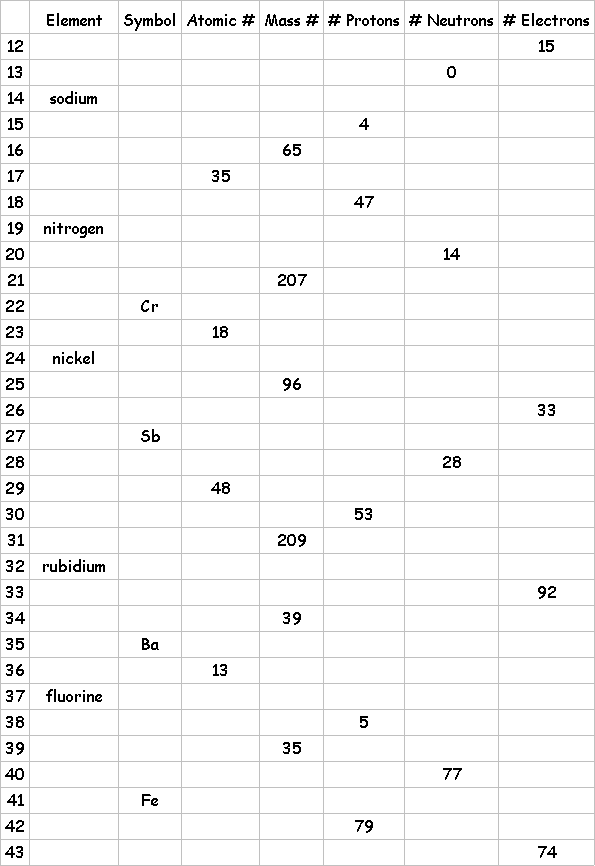
9.The atomic mass number of an element represents the sum of the number of _____________ and the number of ________________ in the nucleus. The symbol for mass number is “A”.
So…#no=A-Z
A=Z+#no
11.Determine
the Z’s and A’s for each of the following elements by looking on a periodic
table.
|
|
Chlorine
|
Magnesium
|
Copper
|
Silver
|
Silicon
|
Xenon
|
|
A
=
|
|
|
|
|
|
|
|
Z
=
|
|
|
|
|
|
|
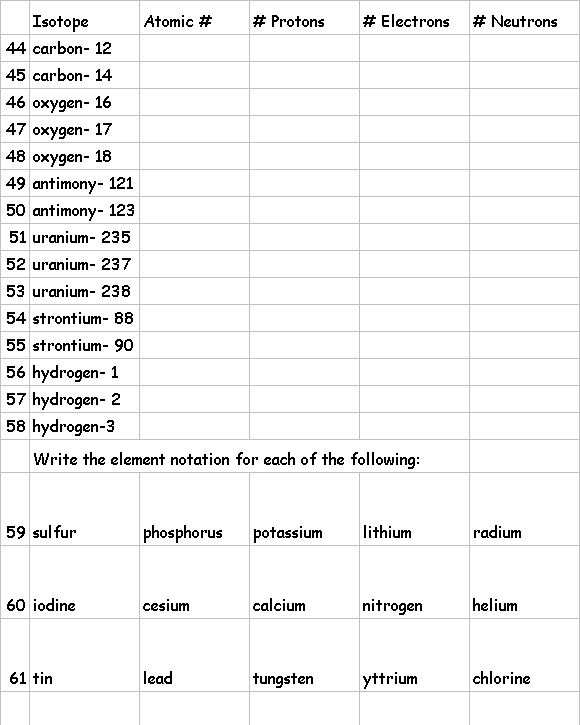
Element
notation is a symbolic way to write an element’s A and Z with the symbol:
![]() The
element notation for sulfur looks like this:
The
element notation for sulfur looks like this:
The
A is to the top left, and the Z is to the bottom left
The
names of isotopes are written with the A following the name.
Complete
the following table:
62.Find
the density of a wood block that has a volume of 5.0 cm3 and
a mass of 0.5 g.
63.What
volume would a rock occupy if it had a mass 31.2 g and a density of 10.4
g/cm3?
64.Calculate
the mass of a wood block that is 4.0 cm long, 2.0 cm wide, 6.0 cm high,
and has a density of 0.50 g/cm3.
65.Which
has the greater mass. 10 cm3 of steel (D = 1.8 g/cm3)
or 5 cm3 of mercury (D = 13.6 g/cm3)?
66.Suppose
you mix: 0.4 liter of red tinted oil (D = 0.90 g/mL) with 0.4 liter of
blue tinted seawater(D = 1.025 g/mL)
in a one liter container. After sealing the container, you shake the mixture
and come back in 1 hour. What do you see in the container?
67.You
have a substance shaped in a cube with a side length = 2.0 cm. The cube's
mass is 24 g. What is the density of this substance?
68.Another
cube of the same substance as the cube in the previous question has a side
length = 4.0 cm. What is the density of this cube?
69.A
jeweler suspects that a piece of gold jewelry in his collection is a fake.
He knows that the density of gold is 19.3 g/cm3. If the volume
of the piece of jewelry is 6.00 cm, and its mass is 109 grams, is the piece
a fake? Why or why not?
70.A
500.0 mL graduated cylinder filled with milk has a mass of 620.0 grams.
The mass of the container is 35 grams. What is the density of the milk?
71.Substances
A and B have the same volume, but the mass of B is twice as great as the
mass of A. How do the densities of the two substances compare?
72.An
irregular-shaped object has a density of 8.0 g/cm3. Its mass
is 72 grams. How many cubic centimeters of water will it displace?
73.A
cube of cork has a density of 0.24 g/cm3. It floats in water.
Suppose you cut a hole in the cork and place lead (D = 11.3 g/cm3)
inside. Now it sinks in water. Why does it sink? Have your changed the
density of the substance cork? What have you changed?
74.How
large a container would you need to hold 195 grams of a liquid that has
a density of1.3 g/cm3?
75.The
density of gasoline is 0.70 g/cm3. Would you use water to put
out a gasoline fire? Why
Solubility and Acids & Bases
77.Give
two examples of each a (solute and a solvent).
78.Describe
how table salt dissolves in H2O.
79.What
factors determine the rate at which a solute dissolves?
80.Briefly
explain how each factor above effects the rate of dissolving for a solute
in a liquid solvent.
81.How
do temperature and pressure effect the solubility of a gas in a liquid?
82.Distinguish
between an unsaturated and a saturated solution.
83.What
is a supersaturated solution?
84.Identify
the solute and the solvent in each of the following. a). ocean water, b).
carbonated water, c) a beaker
full of colored water, d). a sugar water solution
85.How
can you determine whether a solution is saturated, unsaturated, or supersaturated?
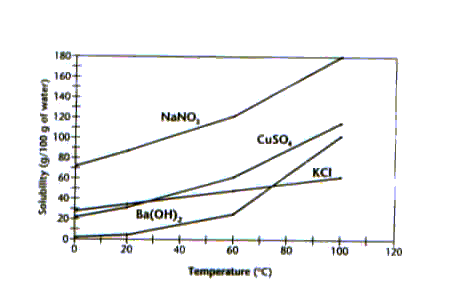
Solubility in g/100 g of Water at the Temperature Indicated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
86.Use
the data table above to determine whether the solutions below are saturated,
unsaturated, or supersaturated.
a.
45.6 g of NH4Cl dissolved in 100g water at 20°C.
b.
0.70 g of PbCl2 dissolved in 100g water at 20°C.
c.
65.3 g of KBr dissolved in 100g water at 20°C.
d.
90.5 g of NaClO3 dissolved in 100g water at 20°C.
87.Give
a definition and an example of an acid.
88.Give
a definition and an example of a base.
89.Describe
what happens in a neutralization reaction?
90.What
does the pH scale measure?
91.Given
the following pH values classify each substance. a). shampoo 5.8, b). blood
7.2, c). ammonia 11.5, d). vinegar 3.0, e). pure water 7.0, f). milk of
magnesia 10.5
92.From
the list in #91, identify which substance is the most basic and which is
the most acidic.
93.From
the list below determine whether the description or property refers to
an acid, a base, or both an acid and a base. Use the following key: A=
Acid B= Base AB= Acid and Base.